Gray Matter Loss in Psychosis is Present in Brain Regions Connected by White Matter
Post by Lani Cupo
The takeaway
Grey matter changes associated with the psychosis spectrum occur in networks connected by white matter, with the hippocampus as a central hub connecting regions of gray matter loss.
What's the science?
The psychosis spectrum, from early first episodes to chronic psychotic disorders like schizophrenia, is associated with gray matter abnormalities, especially atrophy, across the brain. It is yet unknown, however, what mechanism underlies these changes. This week in JAMA Psychiatry Chopra and colleagues demonstrate that gray matter changes are constrained to networks connected by white matter (axonal connections), identifying the hippocampus as a potential source spreading volume loss to different regions.
How did they do it?
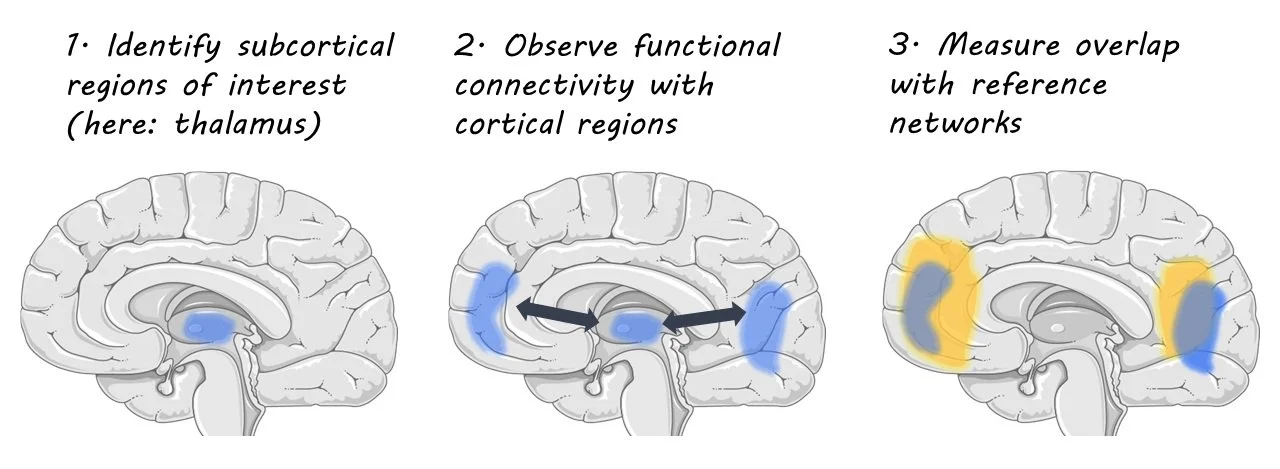
The authors acquired magnetic resonance imaging (MRI) data from 534 people in several groups: patients who had a first episode of psychosis (FEP), but no exposure to antipsychotics, patients who had been exposed to antipsychotics for less than three years, patients with established schizophrenia, and age-matched control groups for each patient group. Comparisons between each patient group and the corresponding control group were made to identify statistical differences in gray matter volume associated with psychotic disorders. In the FEP group, however, additional longitudinal analyses were conducted to isolate the effects of the disorder from those associated with antipsychotics. The authors examined change over time in a control group, patients receiving antipsychotics, and patients receiving a placebo. They could compare changes between the placebo group and control group to identify disorder-related changes and compare changes in the group that received antipsychotics to the placebo group to isolate antipsychotic-related changes. Using images that reflect which brain regions are connected (diffusion weighted imaging and functional MRI), the authors constructed a model of brain networks in a separate healthy dataset. They could then assess whether regions with volume change in the psychosis groups were part of the same brain networks using a coordinated deformation model. Next, the authors used a network diffusion model to assess whether changes in gray matter volume spread evenly throughout the network, or whether certain brain regions served as a hub, or epicenter for volume change.
What did they find?
First, the authors demonstrate that changes in gray matter across stages of illness are constrained by networks connected by white matter. Brain regions that were more strongly connected were more likely to show similar levels of gray matter loss. This suggests changes in various brain regions are not wholly independent, but rather connected in networks. In order to provide evidence that gray matter changes actually spread through axonal connections, however, the authors found that patterns of change associated with both illness and antipsychotic exposure were constrained by brain networks, implying that illness and medication-related changes over time are also related to the connectome. Finally, the authors identified the hippocampus as an epicentre for volume loss, as it was significantly different between patients and controls across all datasets.
What's the impact?
The results of this study suggest gray matter changes associated with psychosis may spread through axonal connections between regions. While there is little evidence for a protein-related spreading of psychosis-related pathology, the cellular profiles of connected regions may share alterations that underlie brain volume changes. Understanding the network-related changes may help future researchers identify the mechanism by which psychopathology impacts the brain to provide better treatment and prevention.