Microglia Rescue Diseased Neurons Via Tunneling Nanotubes
Post by Meredith McCarty
The takeaway
The buildup of protein aggregates in neurons is a feature of many neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease. In this study, it was discovered that tunneling nanotubes connecting microglia and neurons is pivotal in the removal and degradation of these protein aggregates.
What's the science?
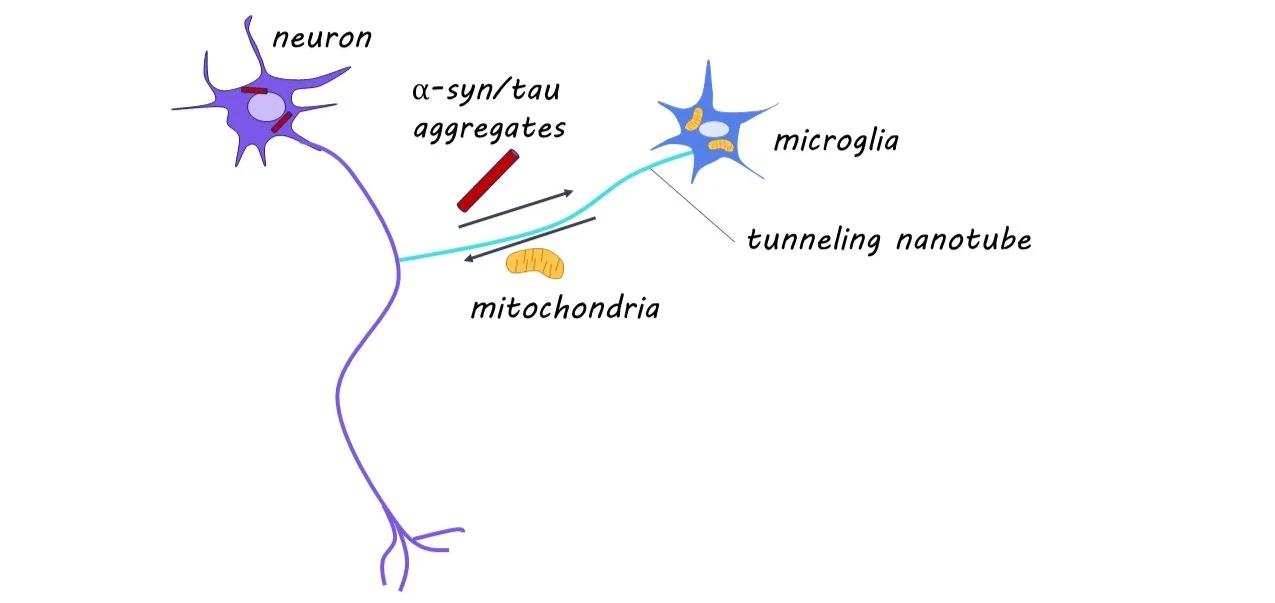
While alpha-synuclein (a-syn) and tau are proteins that perform essential functions in healthy neurons, they can become disruptive and lead to neural damage when they accumulate in the brain. Microglia are immune cells present throughout the brain that clear protein aggregates present in extracellular space and help maintain healthy neural activity. Interestingly, microglia form open channels with neighboring cells, known as tunneling nanotubes (TNTs), to transport protein aggregates and healthy mitochondria (often referred to as ‘the powerhouse of the cell’) to maintain healthy neural function. Before this study, it was unknown whether microglia form TNTs to pathologic neurons to remove protein aggregates.
This week in Neuron, Scheiblich and colleagues find that microglia form TNTs with pathologic neurons, to both remove protein aggregates and deliver healthy mitochondria and that these actions are critical for neural survival.
How did they do it?
They performed RNA sequencing on neurons exposed to protein aggregates to compare how a-syn and tau aggregation affect neuron-microglia interaction. To assess mitochondria levels, they used a fluorescent dye taken in by mitochondria cells. These exposed neurons were cultured along with microglia, and a 3D laser scanning microscopy (a technique that allows reconstruction of 3d structures in the brain) was used to measure TNT formation and quantify the movement of protein aggregates and mitochondria between neurons and microglia. To understand the mechanism of protein transfer through TNTs, they knocked out different components of the Rac-PAK pathway (involved in responding to extracellular signals).
To explore whether TNT formation occurred in human tissue as well, they quantified TNT formation in postmortem human brain tissue from patients with neurodegenerative diseases. The authors used Calcium imaging and patch-clamp experiments when exposed neurons were cultured with or without microglia to measure impairments in neural function. They fluorescently labeled mitochondria, to see whether mitochondria moved from microglia to neurons. They also used an inhibitor of mitochondria to determine whether mitochondria transfer from microglia to neurons was responsible for the improved function in neurons.
They also introduced genetic variants correlated with neurodegenerative diseases to see how these genetic alterations disrupted microglia and neuronal processes.
What did they find?
Through RNA sequencing, they found 951 genes differentially regulated in protein aggregate-exposed neurons. These neurons exhibited reduced levels of mitochondria and increased markers of cell death. They found microglia to be pivotal in the removal of protein aggregates from neurons via TNTs, and that neurons alone cannot degrade protein aggregates. The TNTs formed between microglia and neurons contained protein aggregates, and this transport was found to be unidirectional from affected neurons to microglia. They demonstrate that the Rac-PAK pathway is critical in the removal of protein aggregates via TNTs. When analyzing postmortem human brain tissue, they found TNT formation between neurons and microglia in diseased samples.
Using calcium imaging and patch-clamp methods, they found decreased neural activity in isolated exposed neurons and restored neural activity in exposed neurons cultured with microglia. When co-cultured, they found increased transfer of mitochondria to neurons, and this unidirectional transfer was correlated with improved function in affected neurons. This suggests that the neuroprotective effect microglia have on neurons with high protein aggregation is partly through the transfer of mitochondria via TNTs.
They found that genetic disruption of genes associated with neurodegenerative diseases reduced the protein aggregate removal via TNTs and disrupted the transport of mitochondria from microglia to affected neurons.
What's the impact?
This study illuminates the critical role of TNT connections between microglia and neurons, in both the clearance of protein aggregates from neurons and the transfer of mitochondria to effective neurons. The current findings open the door for much research into how this process may become disrupted in many neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.