Genetic and Environmental Psychiatric Risk Factors Alter Neuronal Projections During Development
Post by Elisa Guma
The takeaway
Exposure to both a genetic and an environmental risk factor for psychiatric illness causes early developmental changes in hippocampal projections to the prefrontal cortex. This structural and functional alteration may underlie many of the cognitive deficits associated with major mental illness.
What's the science?
Projections from the hippocampus to the prefrontal cortex form early in development and support cognitive function. Selective disruption of the connections from the hippocampus to the prefrontal cortex has been associated with poor cognitive function in numerous mental illnesses. The developmental timing of these disruptions has yet to be elucidated. This week in the Journal of Neuroscience, Song and colleagues characterize the structural and functional properties of this pathway during development in the presence of genetic and environmental risk factors in mouse models.
How did they do it?
The mouse model of gene-environment (dual-hit) risk factor was generated by exposing mice carrying a DISC1 allele (heterozygous; genetic risk) to maternal immune activation (environmental risk) early in gestation (gestational day 9, equivalent to the end of the human first trimester). Importantly, DISC 1 mutations have been associated with increased risk for developing schizophrenia, while exposure to maternal immune activation in the womb is a well-recognized risk factor for several neurodevelopmental disorders, including schizophrenia and autism spectrum disorder. Experimental mice of both sexes were tested during neonatal development and pre-juvenile development.
First, the authors aimed to characterize the effect of the dual-hit on the structure of this circuitry. To do so, they performed tracing experiments by injecting a retrograde tracer in the prefrontal cortex and an anterograde tracer in the hippocampus of the mice. Additionally, in a separate group of mice, they exposed embryos to a fluorescent dye that would stain the same circuits. These complementary experiments allowed them to visualize the fibers projection from the hippocampus to the prefrontal cortex at different stages of development.
In order to test the function of these circuits, the authors performed in vivo electrophysiological recording of prefrontal cortex neurons while stimulating the hippocampus optogenetically, in mice. Additionally, in vitro recordings were performed using the patch-clamp technique to record whole-cells in prelimbic and hippocampal neurons to better characterize single-unit activity, firing rate, membrane properties, and synaptic activity of these neurons.
What did they find?
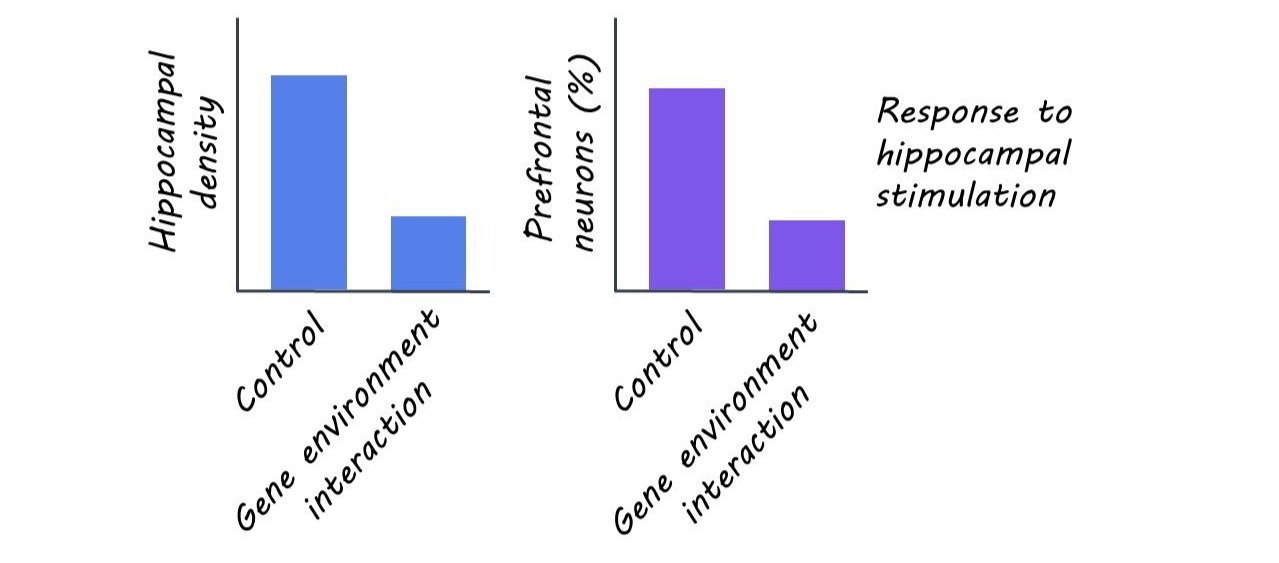
The authors found that neonatal mice exposed to the dual-hit had the same overall pattern of connectivity, but with sparser projections from the hippocampus (specifically from the CA1 region) to the prelimbic area of the prefrontal cortex. Although connectivity increased overall in the pre-juvenile period relative to the neonatal one, these connectivity deficits were found to persist in the dual-hit mice who displayed less arborized (i.e. branched) connections relative to controls.
Given the structural deficits observed due to the dual genetic and environmental risk factor exposure, the authors wanted to determine whether early neuronal function was also disrupted. Overall, they found that stimulation of the hippocampus activated a smaller number of prelimbic neurons in dual-hit mice relative to controls. Further, these mice had decreased occurrence and more variability in their synaptic activity as measured by spontaneous excitatory postsynaptic currents. These results indicate that the sparser hippocampal to prefrontal cortex projections in the dual-hit mice also leads to an attenuated firing rate. As with the structural deficits, the functional deficits observed in the neonates persisted, but to a lesser extent, in pre-juveniles.
What's the impact?
This study suggests that exposure to known genetic and environmental risk factors for psychiatric illness alters connectivity from the hippocampus to the prefrontal cortex, a pathway critical for supporting cognitive function. These changes are detectable at the earliest stages of life, the neonatal period, and persist, albeit to a lesser extent, in the pre-juvenile period. Future work should investigate the direct link between this early disconnection and cognitive impairments, as well as attempt to explore the clinical validity of these developmental mechanisms.