How Are Synapses Eliminated During Development?
Post by Amanda McFarlan
What's the science?
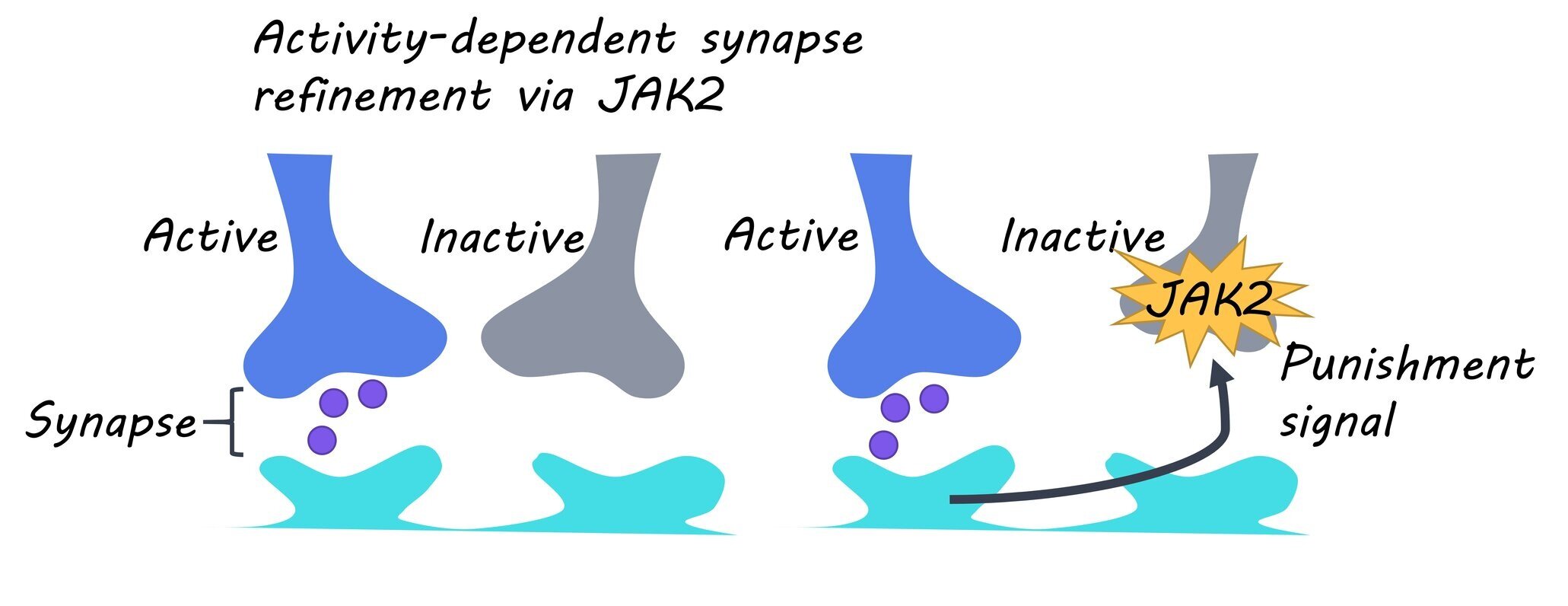
Humans are born with more synaptic connections than needed, and as a result, the brain undergoes a period of synaptic refinement during development where active synapses are stabilized and inactive synapses are eliminated. This process, known as activity-dependent synapse refinement, is critical for the establishment of neural networks in the brain and impairments to this process have been associated with several disorders including schizophrenia and autism. Research has shown that the elimination of inactive synapses requires the competition between inactive and active synapses. However, the molecular signals responsible for this elimination process have yet to be identified. This week in Neuron, Yasuda and colleagues investigated how inactive synapses are eliminated in the brain during development.
How did they do it?
The authors used in utero electroporation to introduce EGFP (a fluorescent protein used for labeling) in callosal neurons - neurons that connect the two cerebral hemispheres of the brain. This labeling allowed them to examine the axon density and to determine the time frame for axonal refinement during development in a mouse model. Then, to investigate whether axonal refinement relies on synaptic activity, the authors suppressed synaptic transmission by introducing tetanus toxin light chain (TTLC) and EGFP in a subset (~20%) of callosal neurons. The authors set out to identify the signaling molecules that are involved in eliminating axons during development. They focused their search on non-receptor protein tyrosine kinases, including JAK2, as these kinases are known to be involved in regulating the maturation of neural networks. Next, the authors used antibody labeling to examine JAK2 activity in inactive neurons during the period of axonal refinement. To determine whether the activation of JAK2 is dependent on signals from other active neurons, the authors quantified JAK2 activity in TTLC-expressing callosal neurons after globally suppressing neural activity with tetrodotoxin. Next, they examined the regulation of JAK2 activity at synapses and the role of JAK2 in the physiological refinement of callosal synapses. Finally, the authors explored whether JAK2 played a role in axonal refinement in other areas of the brain.
What did they find?
The authors found that callosal axons go through a period of refinement from postnatal day 5 (P5) to P15. They showed that compared to controls, the axon density of TTLC-expressing neurons significantly decreased from P10 to P15, suggesting this period of refinement is dependent on synaptic activity. Next, the authors found that axonal elimination observed in TTLC-expressing neurons was inhibited by the expression of a dominant-negative mutant of the JAK2 protein as well as the STAT1 protein (which is activated by JAK2), and thus, they identified JAK2 as the signaling molecule involved in axonal elimination. Then, the authors showed that phosphorylation of JAK2 (indicative of JAK2 activation) was increased in TTLC-expressing callosal neurons compared to controls.
They found that global suppression of neural activity blocked this observed increase in JAK2 phosphorylation, suggesting that the increased activation of JAK2 in inactive neurons is dependent on signals from other active neurons. They then showed that JAK2 is activated at inactive synapses and that JAK2 signaling regulates the physiological refinement of callosal synapses. Finally, the authors determined that JAK2 activation is involved in axon elimination in other areas of the brain, including the refinement of retinal ganglion cell axons.
What’s the impact?
This study identifies JAK2-STAT1 as the key signaling pathway involved in axon and synapse elimination during development. The authors showed that JAK2-mediated elimination of inactive axons and synapses is initiated by signals from other active neurons and occurs in multiple areas of the brain. Together, these findings provide evidence for a signaling pathway that could be targeted for the development of therapeutic treatments for diseases resulting from abnormal synapse refinement such as schizophrenia or Alzheimer’s disease.
Yasuda et al. An activity-dependent determinant of synapse elimination in the mammalian brain. Neuron (2021). Access the original scientific publication here.