Decoding Hippocampal Activity in Spatial Learning
Post by Elisa Guma
The takeaway
Animals need to learn how to find food, water, or other rewarding stimuli in their environment. To learn how to navigate to these rewards, the CA1 region of the hippocampus uses a common learning algorithm by which synchronized activity activates reinforcement learning in both navigational and non-navigational contexts.
What's the science?
Neural activity in the dorsal CA1 region of the hippocampus is critical for our ability to successfully navigate through space, but also for creating cognitive maps of our environment. These neurons are active during navigation, but they also show bursts of brief synchronous activity in non-navigational contexts (e.g., while animals are at rest or asleep), which may reflect memory recall or consolidation, and may suggest a common algorithm for coding reward in both navigational and non-navigational contexts. This week in Nature Neuroscience, Jiang and colleagues investigate the role of the hippocampus in a navigational and non-navigational foraging task by imaging neural activity in the CA1 region of the mouse hippocampus, while they perform these tasks.
How did they do it?
The authors trained mice in either a navigational or non-navigational spatial foraging task while recording neural activity of the dorsal hippocampal CA1 neurons. Mice expressing a fluorescent calcium sensory (CGamp6f) - which fluoresces in the presence of calcium, a proxy for neural activity - were mounted with a miniscope over the dorsal hippocampus, which allowed the authors to record cell activity (fluorescence) during each task.
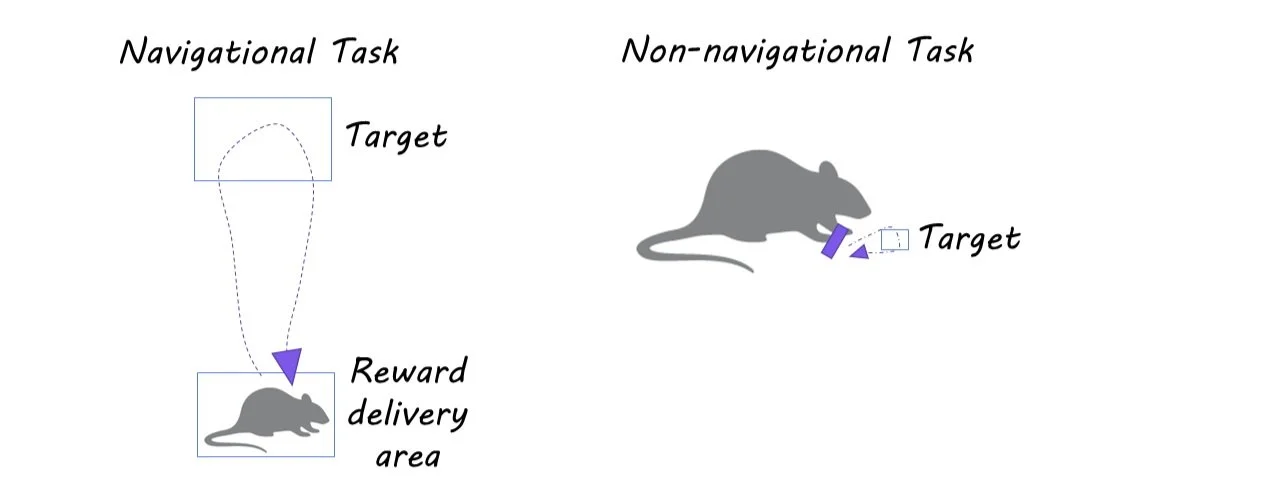
The navigational task required freely moving mice to run to an unmarked target location within a few centimeters of a reward collection area. In the non-navigational task, head-fixed mice had to displace a spring-loaded joystick from a center position to a target distance. In both tasks, reward delivery was dissociated via movement into the target location. In the navigational task, this was accomplished by delivering the reward via a water port at a specific home location (20 cm away from the target location). In the non-navigational task, this was achieved by delivering the water reward with a 1-second delay after movement to the target area.
They examined cell activity during each of the foraging tasks and asked whether responses of individual dorsal CA1 neurons aligned to movements triggered by the water reward. To determine whether patterns of neural activity in the dorsal CA1 region of the hippocampus could predict behaviour, they trained a continuous-time linear decoder (machine learning model) to examine whether movement trajectories could be decoded in both tasks.
The authors identified a specific population of neurons that were synchronously active during non-movement portions of the task, so they wanted to investigate their role in task performance. To test their causal role in consolidating learning in the navigational and non-navigational tasks, the authors used optogenetics to inhibit neural activity in the dorsal CA1 region, at precisely the time that the synchronous population events were observed. A disruption of learning behaviour in response to this manipulation would indicate that the synchronous firing of those neurons was critical for this type of learning.
What did they find?
Using the head-mounted miniscope to measure neural activity in the dorsal CA1, the authors were able to detect place field activity in the dorsal CA1 cells along the foraging trajectories, as expected. In both the freely moving and the head fixed tasks, they found that the reliability of activity was high during movement and low during the inter-trial periods. The neural decoder they had trained was successfully able to predict the foraging trajectory of mice based on the activity of the dorsal hippocampus in the freely moving navigational task. However, in the head-fixed task, it was not able to decode forelimb activity based on CA1 activity, suggesting that these cells may be creating a spatial map of reward locations, not possible in the head-fixed task.
In addition to the cell activity observed during the movement portion of the task, the authors identified a subset of neurons that were synchronously active in the absence of movement. In the freely moving navigational foraging task, these events were observed at the end of a foraging run as the mouse approached the reward collection area, and they were more correlated with correct vs. incorrect attempts (although not significantly so). In contrast, in the non-navigational head-fixed task, these synchronous population events were occurring at the initiation of a trial, and they were highly correlated with the quality of task performance in that trial.
Inactivation of dorsal CA1 cells using optogenetics during the navigation tasks did not interfere with the behaviours. However, if the cells were inactivated during the time at which the synchronous population events were observed (i.e., at the end of the navigational task), trial performance was decreased. In contrast, in the non-navigational task, inactivation with optogenetics substantially reduced the probability of initiating a joystick movement, but if mice were indeed able to initiate movement, they were still capable of making coordinated movements of the joystick to trigger reward. This suggests that these circuits may be more important for navigational learning.
What's the impact?
This study provides evidence for different mechanisms by which the dorsal CA1 region of the hippocampus regulates reinforcement learning in navigational and non-navigational contexts. Activation of synchronous bursts of activity in this region thought to underlie cognitive processing, was associated with successful trial completion if they occurred at the end of the navigational task and at the beginning of the non-navigational task. Interestingly, inactivation of the dorsal hippocampus at the end of a navigational task impaired reinforcement-dependent learning but did not have the same effects in the non-navigational task. This study provides a computational approach to better understand the neural mechanisms underlying spatial navigation and foraging behaviours.