Creating an Atlas to Identify the Neurodevelopmental Stage of Human Brain Tumor Cells
Post by Elisa Guma
The takeaway
By creating an atlas of gene expression in the mouse brain throughout the lifespan, the authors were able to identify expression signatures from adult and pediatric human cerebral tumor development. Brain tumors adopt embryonic-like states, providing insight into the mechanisms by which these tumors grow.
What's the science?
The mechanisms driving cerebral tumor growth are still poorly understood. Within a single tumor, there is high heterogeneity in cell types, many of which resemble various stages of normative neural development. To map the stages of tumor cell development, one must first map normative cerebral development to create a point of reference. This week in Nature Communications, Hamed and colleagues characterize the molecular identity of cells that may drive cerebral tumor cell heterogeneity and determine their relationship to normal developmental states and lineages.
How did they do it?
First, the authors set out to create a comprehensive single-cell atlas of precursor cells (those within the neurogenic and gliogenic compartments) over the course of normal cerebral development. They performed single-cell RNA sequencing of the mouse brain throughout development (11 time points), starting from embryonic day 12.5 (mid-gestation), to postnatal day 365 (1 year old). To identify the appropriate cells to sequence, the authors used a transgenic mouse (Sox2eGFP) which has a fluorescent tag (GFP) on the Sox2 gene that is highly expressed in the cortical neurogenic zones across all stages of prefrontal brain development. Clustering methods were applied to the cells’ transcriptional data to differentiate the identity and developmental signature of the cells.
The second aim of this work was to identify the transcriptional signature of human brain tumor cells. To do so, the authors sequenced human (adult and pediatric) brain tumor cells and used a deconvolution algorithm to map single-cell transcriptomic data acquired from the human brain tumor cells to the mouse clusters they defined above (with a focus on precursor cells). To further validate the reliability of their model the authors aligned human fetal single-cell RNA sequencing data (from a previous publication). They also aligned cells from patient-derived glioblastoma cultures to the mouse clusters identified. Finally, to validate the existence of precursor cells (radial glial precursors) in adult cortical cancer, the authors leveraged a mouse model that uses Nestin-Cre to generate mice with conditional loss of expression of two tumor suppressor genes, the p53 gene (both alleles) and the PTEN gene (one allele) in embryonic precursors. By targeting Nestin+ embryonic precursor cells, the authors were able to gain more central nervous system specificity for tumor development. Indeed, these mice develop glioblastomas postnatally (due to a loss of tumor suppressors) which the authors extracted and sequenced to compare to their single-cell atlas.
What did they find?
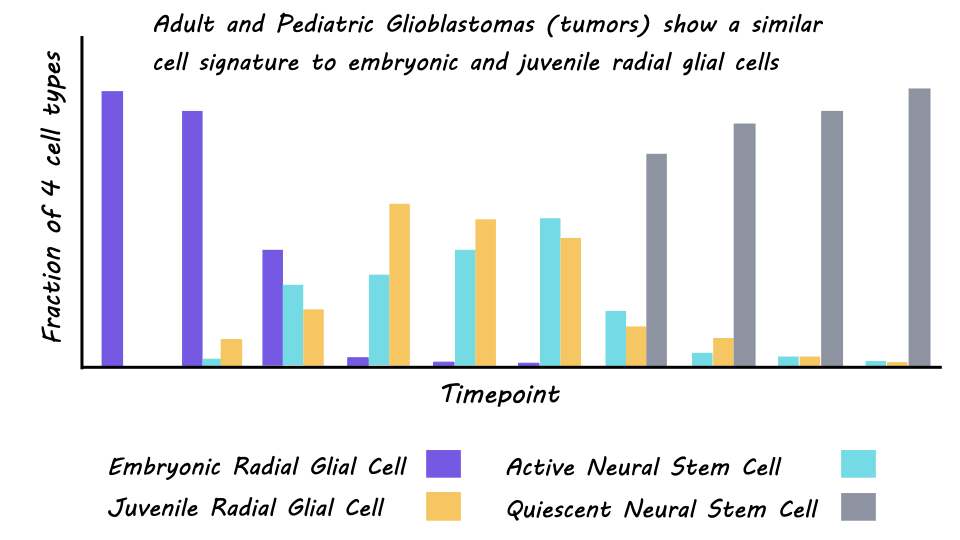
The single-cell atlas of normative brain development comprised 102,504 single cells, with an average of 3,500-13,000 unique molecular identities per cell. These cells could be clustered into three developmental stages: embryonic (mid-late gestation), juvenile (first week of life), and adult (childhood to adulthood). In the embryonic stage, cells were more likely to be undergoing neurogenesis, followed by a switch to gliogenesis which peaked in the juvenile period and continued into young adulthood. Unbiased clustering of the cells also revealed four major clusters with distinct transcriptional profiles. Embryonic radial glial precursor cells denominated the first cluster, juvenile radial glial precursor cells the second, third were glial cells also expressed in the juvenile period, and the fourth was quiescent neural stem cells expressed in the adult period. A deeper characterization of the regional distribution of these cells revealed distinct expression patterns based on the geographical location of the cells within the brain, particularly for the embryonic cells. This incredible resource is publicly available through an app developed by the authors.
The second aim of the study was to use this new atlas to classify the developmental stage of cells from both adult and pediatric brain tumors (glioblastomas). They were able to establish a reliable cross-species mapping between the mouse cells and adult and pediatric human cells, providing another useful resource for the scientific community. Cells from the cerebral gliomas were most similar to those in the embryonic and juvenile stages of the mouse atlas, rather than the adult. This was true for both adult and pediatric tumors. There was also a high similarity between the human fetal cells, cultured glioblastoma cells, and embryonic mouse cells. This both bolsters confidence in the ability of the mouse atlas to characterize cellular heterogeneity in cerebral tumors, and in the embryonic nature of the tumor cells. Finally, malignant tumor cells from the adult mouse brain tumors (from the Nestin-Cre mice with conditional loss of expression of two tumor suppressor genes) also had high similarity to embryonic and juvenile radial glial precursor cells providing further validation for their results.
What's the impact?
This study presents the most comprehensive single-cell atlas of the developing mouse cortex. Furthermore, by achieving reliable cross-species alignment of single-cell transcriptional profiles, the authors were able to use this atlas to classify tumor cells from human adult and pediatric glioblastomas. They found that expression profiles of human tumor cells specifically overlap with embryonic radial glial precursor cells. These findings provide insight into the origins of human brain tumors and aid in our understanding of how normal cells turn into cancer cells. Further, these findings identified potential molecular targets for the treatment of cerebral tumors.