Neuralink’s Brain Chip: Understanding Implantable Brain-Computer Interfaces
Post by Shahin Khodaei
What is Neuralink’s brain chip?
In January 2024, Neuralink, the neurotechnology company owned by Elon Musk, implanted a “brain chip” in a human for the first time. This human is a man named Noland Arbaugh, who has tetraplegia (paralysis in both arms and both legs) due to a spinal cord injury. Neuralink’s coin-sized device was inserted into his skull with the help of a surgical robot, with microscopic wires implanted into the brain tissue to record neural activity. Information from the device is then wirelessly transmitted to a receiving unit for processing. Two months in, the device has given Mr. Arbaugh the ability to use his brain activity to move a computer cursor with enough dexterity to play online chess and the video game Civilization VI.
The Neuralink device is an example of brain-computer interface (BCI) technology. In short, BCIs allow direct communication between the central nervous system (CNS) and a computer. As a result, BCI technologies expand the natural outputs of the CNS, which would normally involve the use of muscles – for example, moving your tongue and mouth to speak, or using your hands to manipulate objects. With BCIs, an entirely new set of artificial outputs from the CNS becomes possible. By directly monitoring brain activity, these devices have been used to type, move a computer cursor, or operate a robotic arm.
What are the components of a BCI system?
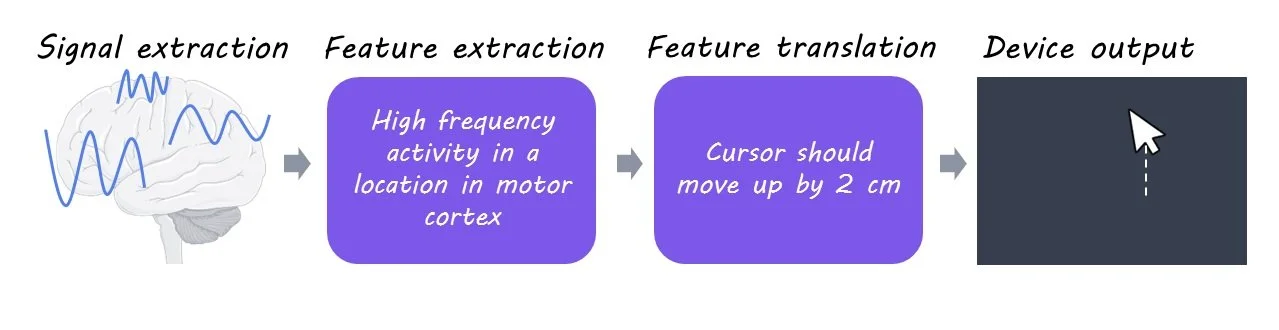
BCI systems consist of four key components: 1) signal acquisition, 2) feature extraction, 3) feature translation, and 4) device output.
1) Signal acquisition: Any BCI system begins by measuring signals from the CNS using sensors. For implanted devices, the sensors are electrodes that are surgically placed under the skull, either on the surface of the brain or penetrating the brain tissue. Brain activity can also be measured in non-invasive ways – for example, by placing sensors on the scalp to measure electrical signals known as the electroencephalogram. In either case, such signals are small in size and therefore need to be amplified, and then converted into a digital format that can be processed by a computer.
2) Feature extraction: Signals recorded from the CNS are rich with information. However, not all the information within the signal is relevant for a particular BCI. In this component, the relevant features of the signal are extracted for further processing. The extracted feature should have a strong correlation with the user’s intentions. For an implanted device, the extracted feature is often the activity pattern of groups of neurons around the sensors.
3) Feature translation: The extracted features are then given to a translation algorithm. This algorithm is designed to translate the relevant features of the signal into commands that reflect the user’s intent. For example, a specific activity pattern may be translated to “move the computer cursor upward”, and another pattern to “move the computer cursor downward”. In this way, the user’s goal is deduced from their brain activity.
4) Device output: The commands from the translation algorithm go on to operate an external device. Depending on the nature of the BCI system, the final output may be the movement of a computer cursor, the operation of a robotic arm, steering an electric wheelchair, etc.
In some instances, there may be a fifth component, where the BCI system delivers input back to the brain to modulate the CNS. This input may be delivered by directly applying electrical currents into the brain tissue through the implanted electrodes, or by non-invasive methods such as transcranial magnetic stimulation.
Progress in BCI technology
Major advances have been made since the first report of BCI technology in the 1960s when electrical signals recorded from the scalp were used to control a slide projector. The main goal of BCI research and development so far has been to assist people affected by stroke, spinal cord injury, or CNS disorders such as amyotrophic lateral sclerosis. The most common use of BCIs has been to replace natural CNS output that is lost to injury, which was reported as early as 2006. In that year, Hochberg et al. published a study in Nature reporting that a patient with tetraplegia (similar to Mr. Arbaugh) used an implanted BCI device to control both a computer cursor and a robotic arm.
Hochberg’s 2006 device implanted 96 electrodes into the participant’s brain. Nearly 20 years later, the BCI implant from Neuralink can measure brain activity using more than 3000 electrodes placed in brain tissue. This represents a significant improvement in the signal acquisition component of BCIs. With ongoing innovations in machine learning, the signals can be also processed in new ways for better feature extraction and translation. Together, these technologies will likely advance the capabilities of BCIs.
There have also been exciting uses for BCIs to not just replace, but to restore natural CNS output. In a 2023 study published in Nature, researchers developed a BCI system where the device output was electrical stimulation of the spinal cord. Specifically, the stimulation activated areas of the spinal cord that controlled muscles involved in walking. Using this strategy in a participant with tetraplegia, their device translated brain activity into leg movements to restore the participant’s ability to walk.
BCIs also have the potential to either enhance or supplement the natural outputs of the CNS. In this way, researchers have explored how BCIs can help the general population. For example, a BCI can improve performance in tasks that require intense concentration by detecting brain activity that indicates loss of attention and playing a sound to restore concentration.
Potential risks associated with BCI devices
Often, implanted BCI devices require an invasive and high-risk open brain surgery to place sensors into the brain. There is an unavoidable risk of damage to the brain area where the implant is placed, as well as possible complications such as infection, bleeding, and brain swelling. While these risks may be acceptable for users with severe disabilities who can greatly benefit from implanted BCIs, they discourage most individuals from getting an implant. It is worth mentioning that newer minimally invasive BCI devices are currently being developed. An example of this is the Stentrode sensor developed by Synchron. Instead of open brain surgery, the Stentrode is inserted into the brain through the jugular vein, using a minimally invasive endovascular surgery.
If and when BCIs become more broadly used, there will be a growing risk to user’s privacy and safety. These devices are likely to measure brain activity in increasing detail as the hardware and software evolve. If the recordings of brain activity are not immediately discarded, it is critical that they are stored safely and privately. This is particularly important as the BCI field attracts more private companies, whose business interests may not align with the user’s expectation of data privacy. Additionally, the digital components of the BCI system, in particular the device output, may be vulnerable to threats such as hacking.
Takeaway
Implanted BCI technologies show great potential in assisting individuals with neurological injury or disease. As the tools evolve to improve all components of BCI systems, BCIs could become an important technology to not only replace and restore function for people with disabilities but also to enhance and supplement performance in the general population.
References +
He et al. Brain–computer interfaces. 2020. Neural Engineering. Access the publication here.
Hochberg et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006. Access the publication here.
Lorach et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature. 2023. Access the publication here.
Maiseli et al. Brain-computer interfaces: trend, challenges, and threats. Brain Informatics. 2023. Access the publication here.
Mitchell et al. Assessment of safety of a fully implanted endovascular brain-computer interface for severe paralysis in 4 patients: the Stentrode with thought-controlled digital switch (SWITCH) study. 2023. JAMA Neurology. Access the publication here.
Musk and Neuralink. An integrated brain-machine interface platform with thousands of channels. Journal of Medical Internet Research. 2019. Access the publication here.
Oi. Neuralink: Musk’s firm says first brain-chip patient plays online chess. 2024. BBC. Access the publication here.
Shih et al. Brain-computer interfaces in medicine. Mayo Clinic Proceedings. 2012. Access the publication here.