Identifying an fMRI Biomarker for Cognitive Decline in Alzheimer’s Disease
Post by Kelly Kadlec
The takeaway
Two fMRI-based metrics previously used to evaluate cognitive decline with age may also be useful for assessing both the risk and severity of Alzheimer’s disease. These scores can help distinguish between rates of memory decline in healthy individuals and those with varying levels of risk for developing Alzheimer’s.
What's the science?
A formal diagnosis of Alzheimer’s disease (AD) is often preceded by progressive stages of cognitive decline. At each of these stages, patients are at varying risk for advancing to AD, but assessing the risk of an individual is difficult due to a high degree of heterogeneity in neurocognitive aging. Previously, functional magnetic resonance imaging (fMRI) contrast maps for novelty and memory tasks have yielded two corresponding single-value scores that have been proposed as biomarkers of neurocognitive aging. This week in Brain, Soch and colleagues compare these fMRI-based scores in healthy individuals, individuals with AD, and individuals in different risk categories for developing AD, to assess their ability to distinguish between clinical and healthy rates of cognitive decline.
How did they do it?
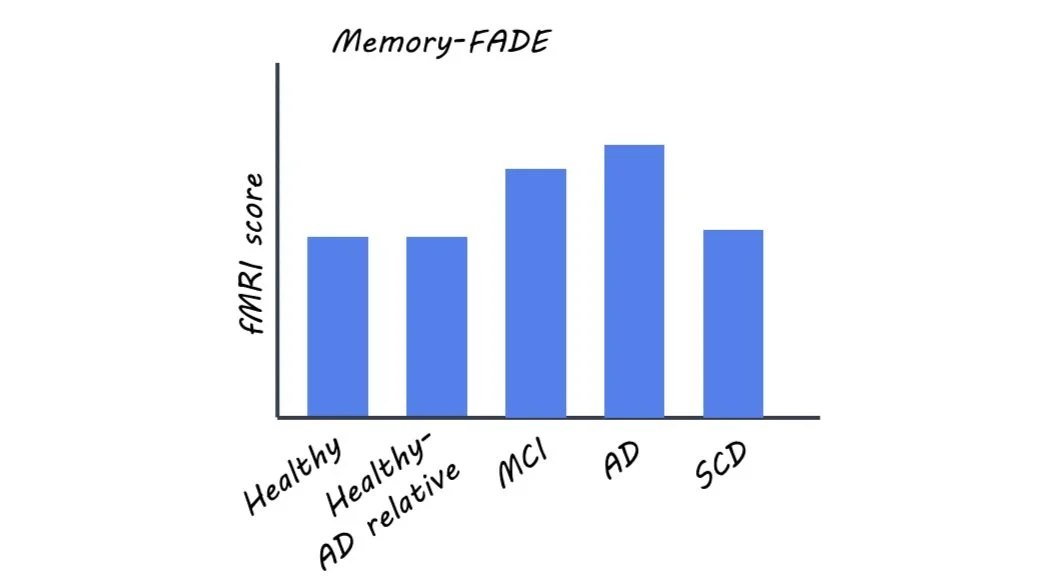
This study comprised five groups of individuals: healthy controls with no family history of AD, healthy individuals with a first-degree relative with AD, patients with AD, and patients in one of two symptom-based risk states for AD: mild cognitive impairment (MCI) or subjective cognitive decline (SCD), where MCI is considered the more severe.
The authors collected fMRI data from the participants during image-based novelty and memory tasks. They used the resulting contrast maps to calculate two scores: Functional Activity Deviation during Encoding (FADE) and Similarity of Activations during Memory Encoding (SAME). Additionally, psychometric and genetic testing was done for each participant, and a subset had amyloid positivity testing. The authors hypothesized that increasing FADE scores and decreasing SAME scores would be associated with worse AD severity and higher risk for AD.
What did they find?
The authors found that memory and novelty-based FADE and SAME scores could be used to distinguish between the different participant groups and also correlated with known risk factors and cognitive assessments.
The authors reported that increasing risk for AD corresponded to larger deviations in FADE and SAME scores (i.e. more atypical fMRI results). Only memory-based FADE and SAME scores differentiated between the two more severe clinical groups (AD and MCI) and all other participant groups, and only novelty-based scores distinguished between AD and MCI patients.
The authors confirmed that FADE and SAME scores for memory and novelty tasks corresponded to other currently used psychometric tests of cognitive decline and AD severity. The authors also found that within the AD-related participants, higher FADE and lower SAME scores corresponded to the presence of an AD genotype. In addition, the authors found that especially novelty-based scores were sensitive to amyloid positivity.
To demonstrate the potential clinical value of these fMRI biomarkers, the authors used FADE and SAME scores to predict diagnostic groups for the participants and classified each group with above-chance accuracy. They also used these scores to predict the presence of an AD genotype in participants with AD relatives.
What's the impact?
Proper assessment of AD risk and severity is challenging, and this study proposes two promising neural biomarkers. These fMRI-based scores distinguished between differing stages of the disease and predicted other proposed risk factors for AD. This knowledge is critical for choosing the correct treatment routes and improving diagnosis accuracy earlier in the development of AD. Further, a longitudinal study is needed to determine how predictive they are of future outcomes (i.e. MCI progressing to AD).