Ripples During Slow-Wave Sleep Re-balance Neurons
What's the science?
Synapses in the brain are strengthened while awake and synaptic depression (weakening of the synapses) occurs during slow-wave sleep to rebalance synapses. This synaptic depression may help to selectively preserve memories, however, how it occurs during sleep is unclear. During slow-wave sleep, the hippocampus emits high frequency oscillations called “sharp-wave ripples” which reactivate the neurons involved in recent memories. Ripples could be required for synaptic depression during sleep, making “room” for new memories to form. This week in Science, Norimoto and colleagues test whether ripples are required for synaptic depression and subsequent memory formation in mice.
How did they do it?
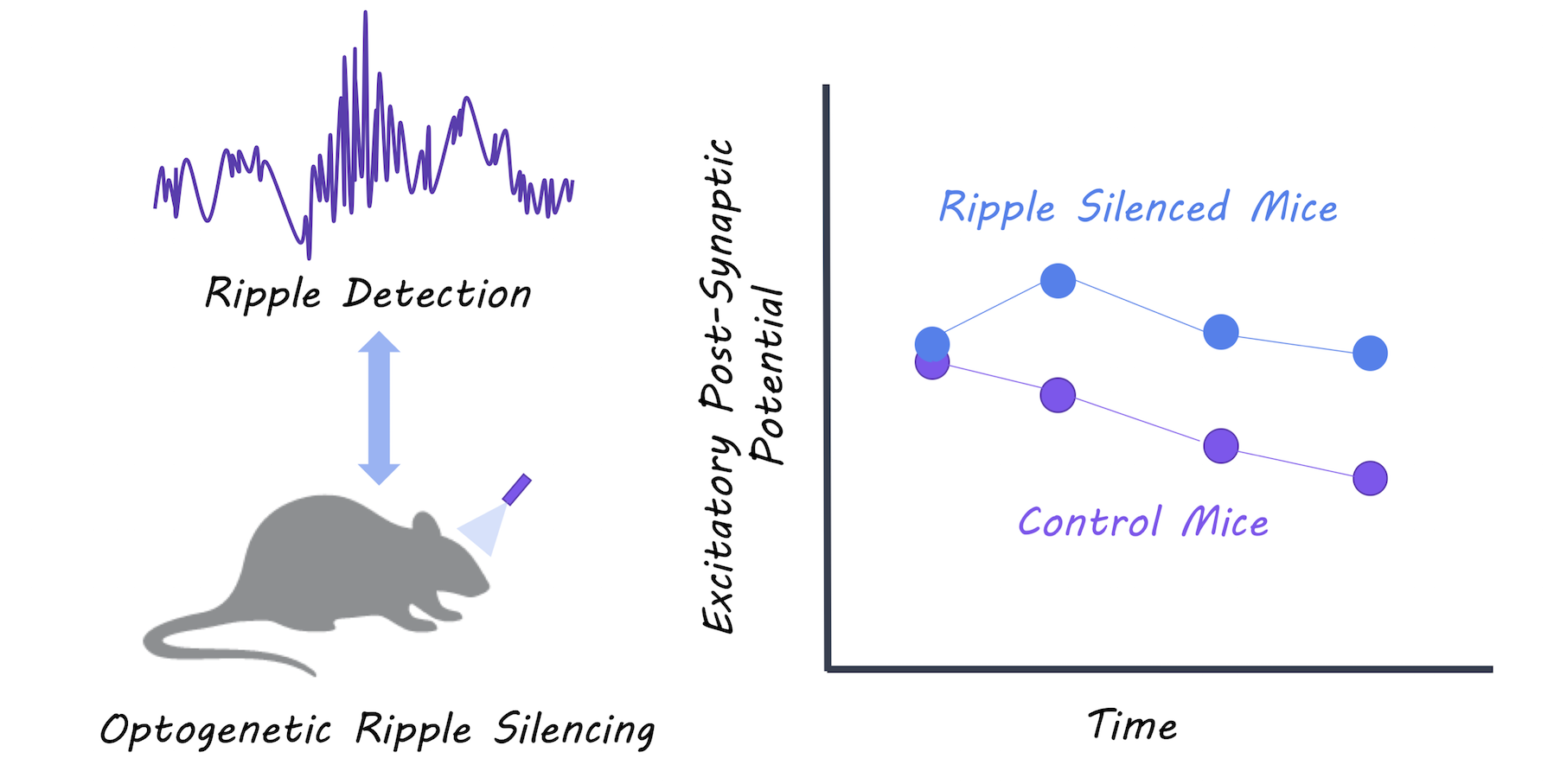
They silenced sharp-wave ripple events during slow-wave sleep using optogenetic-feedback (i.e. every time a ripple event was detected, they inhibited the hippocampal neurons to reduce firing) in a group of test mice. They then recorded excitatory postsynaptic potentials (activity in the postsynaptic neurons) during slow-wave sleep and compared the test mice to control mice to determine whether synaptic depression during sleep was affected in the test mice. Mice then underwent a spatial object-recognition task. First, they explored an area containing two new objects, and then returned to the same location 2 hours later where one of the objects had moved. The authors compared the memory performance between test and control mice to determine whether disrupting the ripples had an effect on new memory acquisition.
What did they find?
The authors were able to successfully reduce almost all of the ripple events in the test mice. Control mice experienced depressed postsynaptic activity representing the expected synaptic depression that occurs during slow-wave sleep, while mice with the impaired ripple events did not show synaptic depression. Control mice showed normal memory performance, while mice with impaired ripple events were unable to identify a moved object in the spatial object recognition task, suggesting that they could not form new memories. The authors were also able to replicate the lack of synaptic depression by testing the effects of disrupting ripples in hippocampal tissue slices. They used in vitro and in vivo experiments to show that synaptic depression occurring during slow-wave sleep is mediated by NMDA receptors.
What's the impact?
This is the first study to link sharp-wave ripples during slow-wave sleep with synaptic depression and memory performance. Before this study, we did not understand the mechanism through which synaptic depression occurred during slow-wave sleep. We now know that ripples during slow-wave sleep are critical for balancing of synapses and for new memory formation.
H. Norimoto et al., Hippocampal ripples down-regulate synapses. Science (2018). Access the original scientific publication here.